
Zinc Protein Research
The Role of Metal-Ion Binding in Protein Folding
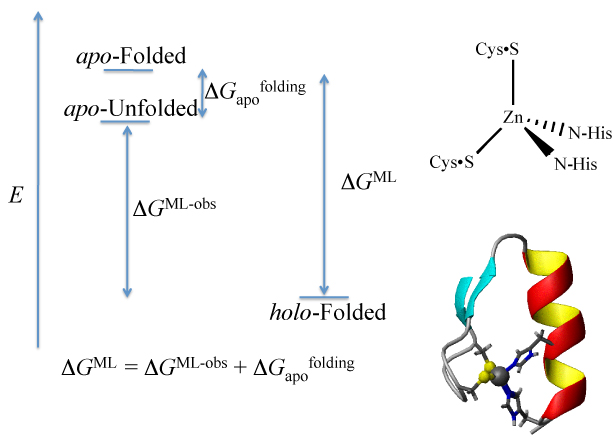
Zinc finger proteins(ZFPs), the largest single class of metalloproteins in the human genome, regulate gene transcription and, therefore, protein expression, by site-specific binding to DNA and RNA. With respect to ZFPs involved in cancer, the Gibney laboratory is focused on understanding the thermodynamic role of Zn(II)-binding in protein folding, i.e. metal-induced protein folding, and its relationship to nucleic acid binding. Our approach is to determine the formation contants for zinc-binding peptide and proteins from equilibrium measurements taken with a suite of spectroscopic and calorimetric methods. This approach demonstrates that the thermodynamic cost of protein folding in ZFPs with metal-induced protein folding events is relatively small (0-5 kcal/mol) compared to the thermodynamic contribution of Zn(II)-binding (15 kcal/mol). Our long-term goal is to improve the rational design of artificial ZFP therapeutics by characterizing the fundamental thermodynamics of their interactions with Zn(II) metal-ions and DNA.
The Gibney Lab currently evaluating the formation constants of Zn(II) binding to human transcription factor IIB, which is stably folded prior to Zn(II) binding. This system should provide critical insight into the free energy required to fold ZFPs.

